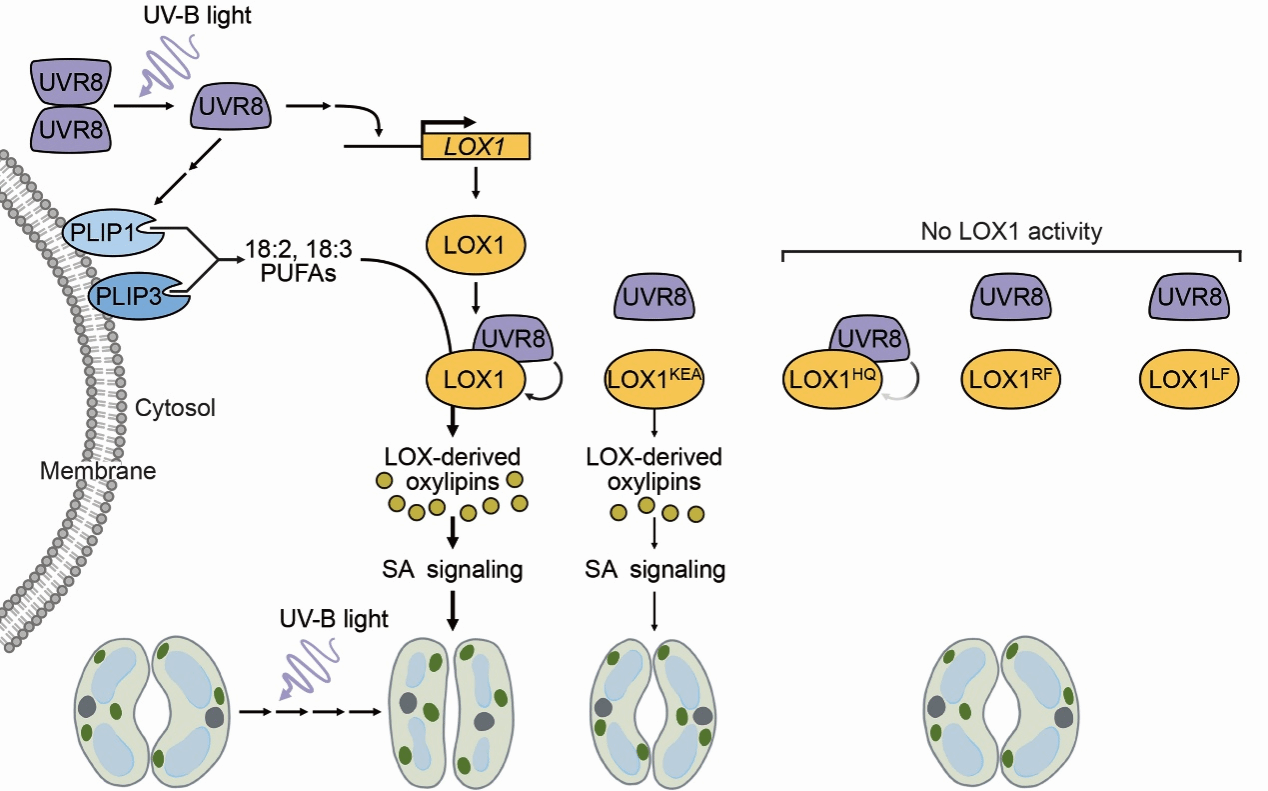
Abstract: Ultraviolet-B (UV-B) light-induced stomatal closure requires the photoreceptor UV RESISTANCE LOCUS 8 (UVR8) and nitric oxide (NO). However, the signaling pathways by which UV-B light regulates stomatal closure remain elusive. Here, we reveal that UVR8 signaling in the epidermis mediates stomatal closure in a tissue-specific manner in Arabidopsis (Arabidopsis thaliana). UV-B light promotes PHOSPHOLIPASE 1 (PLIP1)/PLIP3-mediated linoleic acid and α-linolenic acid accumulation and induces LIPOXYGENASE 1 (LOX1) expression. LOX1, which catabolizes linoleic acid and α-linolenic acid to produce oxylipin derivatives, acts downstream of UVR8 and upstream of the salicylic acid (SA) pathway associated with stomatal defense. Photoactivated UVR8 interacts with LOX1 and enhances its activity. Protein crystallography demonstrates that A. thaliana LOX1 and its ortholog in soybean (Glycine max) share overall structural similarity and conserved residues in the oxygen cavity, substrate cavity, and metal-binding site that are required for 9-LOX activity. The disruption of UVR8–LOX1 contact sites near the LOX1 oxygen and substrate cavities prevents UVR8-enhanced LOX1 activity and compromises stomatal closure upon UV-B exposure. Overall, our study uncovers a noncanonical UV-B signaling module, consisting of the UVR8 photoreceptor and the cytoplasmic lipoxygenase, that mediates stomatal responses to UV-B light.
Link: https://doi.org/10.1093/plcell/koaf060