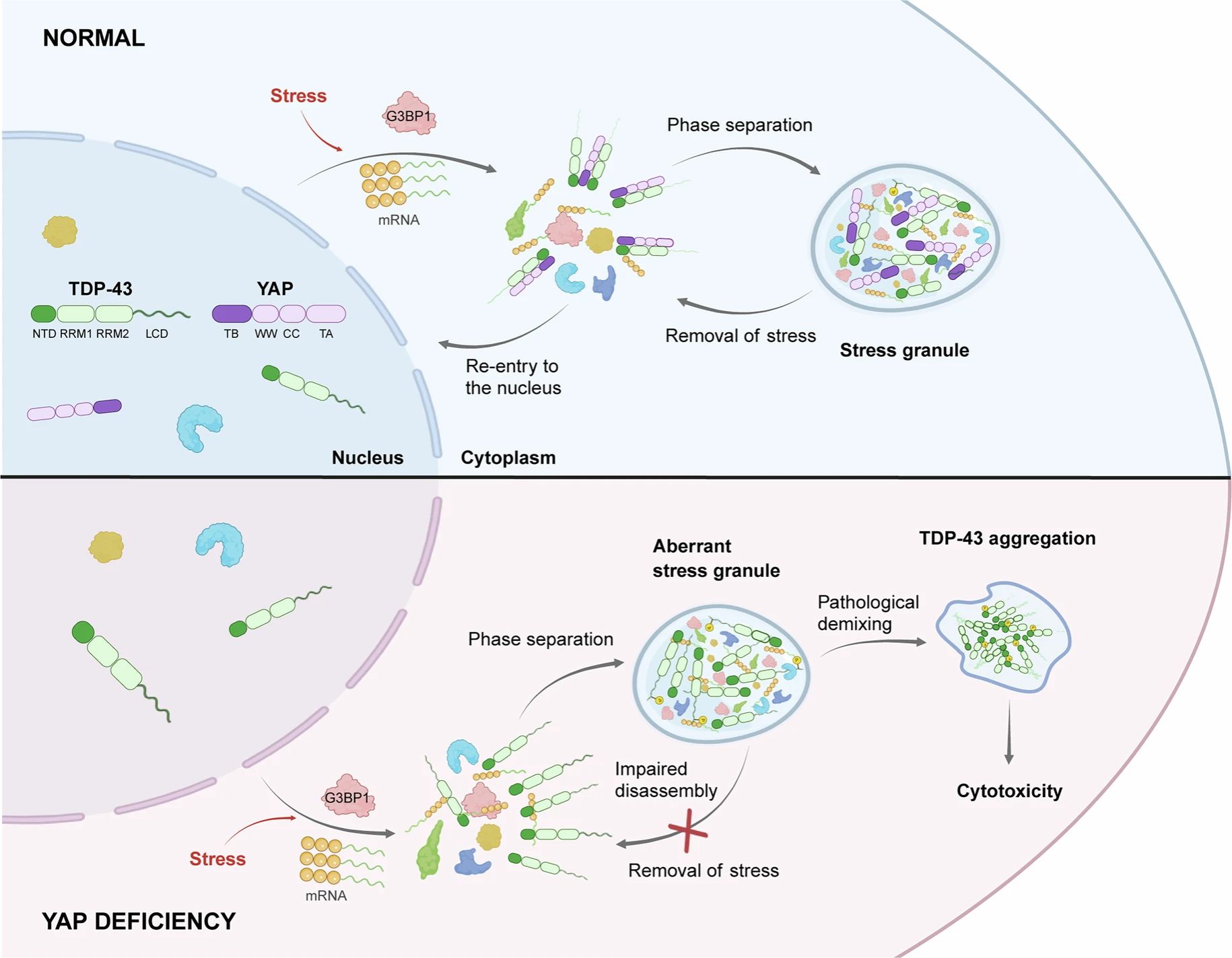
Abstract: Recent studies exploring the underlying pathomechanisms of amyotrophic lateral sclerosis (ALS), a fatal motor neuron disorder, have focused on biomolecular condensates. Here we reveal an unexpected function for YAP, a central component of the Hippo pathway, in regulating the dynamic behaviour of stress granules and TDP-43 condensates, a role that is independent of its transcriptional activity in the Hippo pathway. YAP directly binds to TDP-43. This interaction directly promotes the homotypic multimerization and phase separation of TDP-43 while inhibiting its hyperphosphorylation and solidification under stress conditions. Remarkably, YAP, whose messenger RNA levels are reduced in patients with ALS, is found to co-localize with pathological hyperphosphorylated TDP-43 aggregates in the brains of patients with ALS. In addition, elevation of YAP/Yorkie (a fly homologue of mammalian YAP) expression substantially reduces TDP-43 toxicity in primary neuron and transgenic fly models of ALS. Our findings highlight an unexpected role of YAP in managing ALS-associated biomolecular condensates, presenting important implications for potential ALS treatments.
Link: https://doi.org/10.1038/s41556-025-01685-y