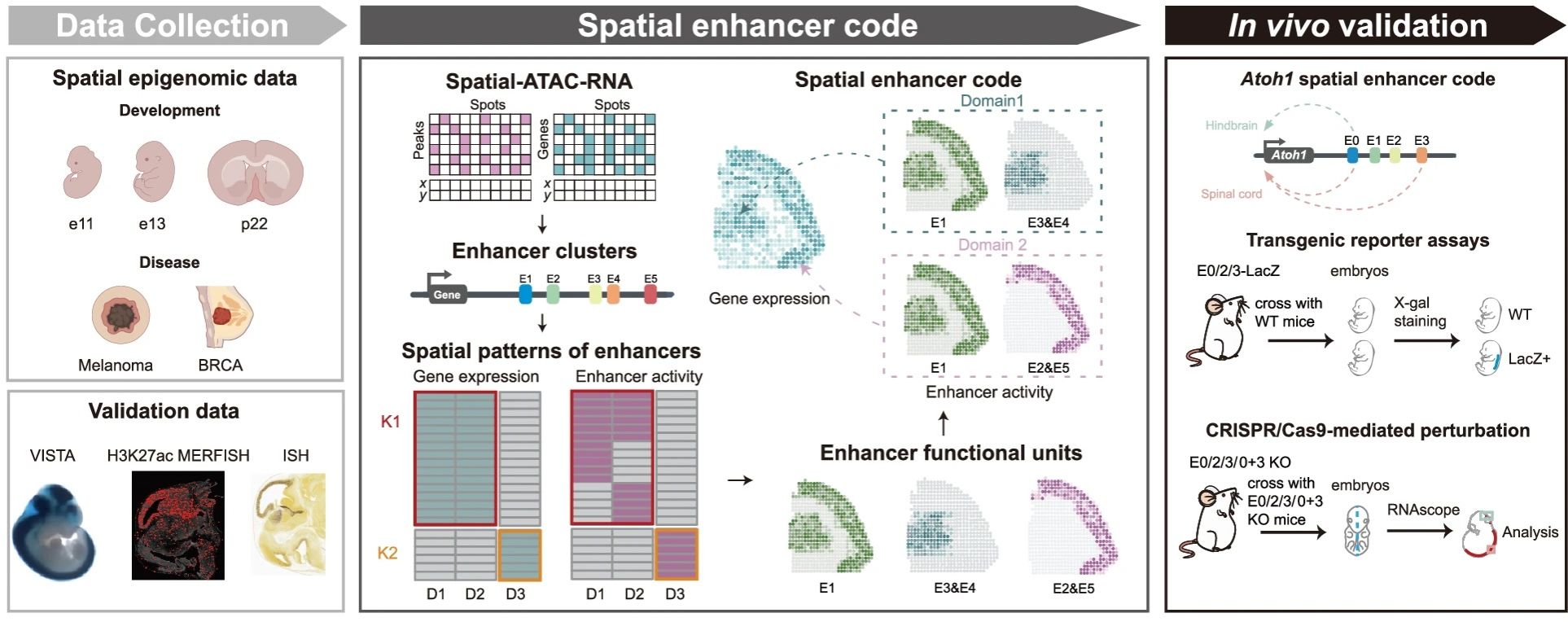
Abstract: Spatial transcriptomics and epigenomics have enabled mapping gene regulation in the tissue context. However, it remains poorly understood how spatial gene expression patterns are orchestrated by enhancers. Here we build eSpatial, a computational framework that deciphers spatially resolved enhancer regulation of gene expression by integrating spatial profiles of gene expression and chromatin accessibility. Applying eSpatial to diverse spatial datasets, including mouse embryo and brain, as well as human melanoma and breast cancer, we reveal a “spatial enhancer code”, in which divergent combinations of enhancers regulate the same gene in spatially segregated domains. We validate the spatial enhancer code using public spatial datasets such as VISTA, Allen in situ hybridization (ISH), and H3K27ac MERFISH. Moreover, we conduct transgenic reporter assays and in vivo CRISPR/Cas9-mediated perturbation experiments to confirm the Atoh1 spatial enhancer code in determining Atoh1 spatial expression in mouse embryonic spinal cord and brain. Our study establishes the spatial enhancer code concept, revealing how combinations of enhancers dynamically shape gene expression across diverse biological contexts, providing insights into tissue-specific regulatory mechanisms and tumor heterogeneity.
Link: https://doi.org/10.1038/s41467-025-60482-1