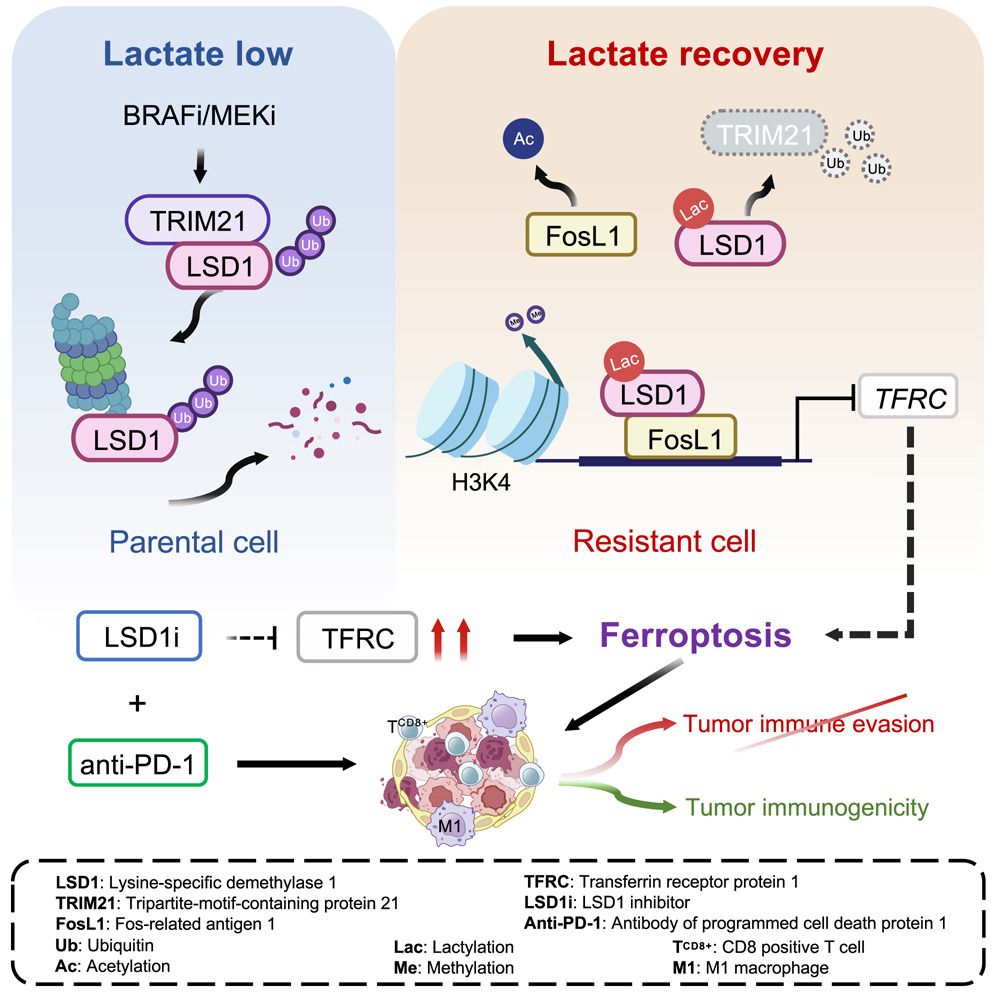
Abstract: BRAFV600E mutant melanomas treated with BRAF inhibitor (BRAFi) and MEK inhibitor (MEKi) almost invariably develop drug resistance, accompanied by restored glucose metabolism. How resumed glycolysis controls acquired resistance remains unknown. Here, we identify that lysine-specific demethylase 1 (LSD1) lactylation, induced by re-accumulated lactate in both human and murine BRAFi/MEKi-resistant melanoma cells, selectively drives survival via epigenetic reprogramming. Mechanistically, lactylation of LSD1 promotes its interaction with Fos-related antigen 1 (FosL1), preventing its degradation by E3 ligase tripartite-motif-containing protein 21 (TRIM21) and selectively enhancing its genomic enrichment. We further demonstrate that lactylated LSD1 co-directs gene transcription with FosL1 to repress ferroptosis via interfering with transferrin receptor protein 1 (TFRC)-mediated iron uptake. LSD1 inhibition activates ferroptosis, resulting in drastic regression of drug-resistant murine melanoma when combined with immunotherapy. Our results highlight a crucial role of metabolic rewiring-induced epigenetic reprogramming as a bypass resistance mechanism in BRAFi/MEKi-resistant melanoma, providing a therapeutically actionable strategy to overcome resistance to targeted therapy and immunotherapy.
Link: https://doi.org/10.1016/j.devcel.2025.02.016